Pd-catalyzed gem-difluoroallylation of arylboronic acids with γ,γ-difluoroallylic acetates†
Abstract
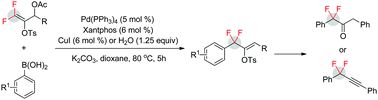
A highly regio- and stereo-selective palladium-catalyzed gem-difluoroallylation of arylboronic acids with γ,γ-difluoroallylic acetates has been described. The method allows the synthesis of a variety of gem-difluoroallylated arenes with a tosyloxy group on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, thus providing a good opportunity for down-stream transformations.
C double bond, thus providing a good opportunity for down-stream transformations.

 Please wait while we load your content...
Please wait while we load your content...