Expanding the scope of alkyne-mediated bioconjugations utilizing unnatural amino acids†
Abstract
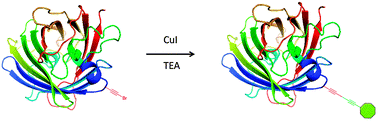
The importance of bioconjugates within the field of chemistry drives the need for novel methodologies for their preparation. Well-defined and stable bioconjugates are easily accessible via the utilization of unnatural amino acids (UAAs). As such, we have synthesized and incorporated two new UAAs into green fluorescent protein, and optimized a novel Cadiot–Chodkiewicz bioconjugation, effectively expanding the toolbox of chemical reactions that can be employed in the preparation of bioconjugates.

 Please wait while we load your content...
Please wait while we load your content...