Carbamoyl anion-initiated cascade reaction for stereoselective synthesis of substituted α-hydroxy-β-amino amides†
Abstract
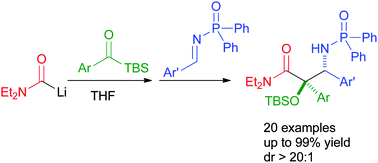
A carbamoyl anion-initiated cascade reaction with acylsilanes and imines has been used to rapidly construct substituted α-hydroxy-β-amino amides. The Brook rearrangement-mediated cascade allows the formation of two C–C bonds and one O–Si bond in a single pot. Using this approach, a range of α-aryl α-hydroxy-β-amino amides has been synthesized in high yields with excellent diastereoselectivities.

 Please wait while we load your content...
Please wait while we load your content...