Distinction between coordination and phosphine ligand oxidation: interactions of di- and triphosphines with Pn3+ (Pn = P, As, Sb, Bi)†
Abstract
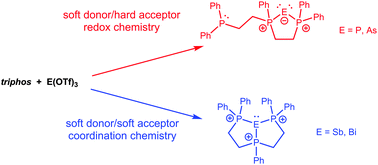
Reactions of polydentate phosphines with sources of Pn3+ (Pn = P, As, Sb, Bi) yield complexes of Pn1+ (Pn = P, As) or Pn3+ (Pn = Sb, Bi) acceptors. The distinction between coordination of a phosphine center to Pn and oxidation of a phosphine ligand is dependent on Pn. The first structurally verified triphosphine complexes of Sb(III) and Bi(III) acceptors are reported.

 Please wait while we load your content...
Please wait while we load your content...