Unified synthesis of tirandamycins and streptolydigins†
Abstract
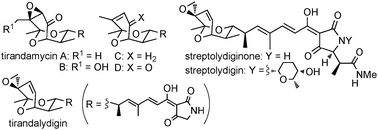
The asymmetric total syntheses of tirandamycins A–D and tirandalydigin as well as the synthesis of the left-hand fragment of streptolydiginone and streptolydigin from a common intermediate are described. The comprehensive approach features the highly enantio- and diastereoselective assembly of the anti,anti,syn-stereotetrad unit which relies on a cinchona alkaloid-catalyzed asymmetric Morita–Baylis–Hillman reaction.

 Please wait while we load your content...
Please wait while we load your content...