Uniaxial magnetic anisotropy of square-planar chromium(ii) complexes revealed by magnetic and HF-EPR studies†
Abstract
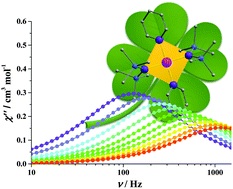
Two mononuclear square-planar Cr(II) complexes are reported, exhibiting field-induced slow magnetic relaxation. The axial zero-field splitting parameter was unambiguously determined by both a high-frequency/field electron paramagnetic resonance (HF-EPR) technique and magnetic measurements. This result represents the first observed single-molecule-magnet behavior in the square planar coordination geometry of any metal ions.

 Please wait while we load your content...
Please wait while we load your content...