Benzylic C–H trifluoromethylation of phenol derivatives†
Abstract
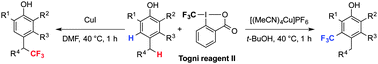
Phenol derivatives were trifluoromethylated using copper/Togni reagent. In dimethylformamide, the benzylic C–H bond at the para position of the hydroxyl group was selectively substituted with a CF3 group. In contrast, aromatic C–H trifluoromethylation occurred in alcoholic solvents. Practical utility of the reactions was demonstrated by application to the synthesis of a potent enoyl-acyl carrier protein reductase (FabI) inhibitor.

 Please wait while we load your content...
Please wait while we load your content...