Geminal bis(silane)-controlled regio- and stereoselective oxidative Heck reaction of enol ethers with terminal alkenes to give push–pull 1,3-dienes†
Abstract
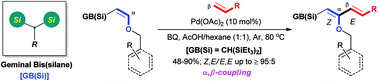
A geminal bis(silane)-controlled regio- and stereoselective oxidative Heck reaction of enol ethers with terminal alkenes has been developed. The reaction proceeds with α,β-coupling regioselectivity to give push–pull Z,E-1,3-dienes in good yields. The product showed valuable utility in Sakurai homoallylation with acetals to generate α-substituted-γ-keto esters with good anti-selectivity.

 Please wait while we load your content...
Please wait while we load your content...