Triphosgene–pyridine mediated stereoselective chlorination of acyclic aliphatic 1,3-diols†
Abstract
We describe a strategy to chlorinate stereocomplementary acyclic aliphatic 1,3-diols using a mixture of triphosgene and pyridine. While 1,3-anti diols readily led to 1,3-anti dichlorides, 1,3-syn diols must be converted to 1,3-syn diol monosilylethers to access the corresponding 1,3-syn dichlorides. These dichlorination protocols were operationally simple, very mild, and readily tolerated by advanced synthetic intermediates.
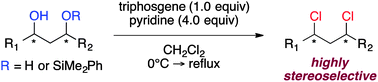

 Please wait while we load your content...
Please wait while we load your content...