The asymmetric synthesis of polycyclic 3-spirooxindole alkaloids via the cascade reaction of 2-isocyanoethylindoles†
Abstract
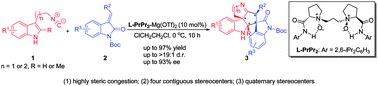
A highly enantioselective dearomative cascade reaction between 2-isocyanoethylindoles and 3-alkenyl-oxindoles was realized using a chiral N,N′-dioxide–Mg(II) complex catalyst. This reaction provides a straightforward access to polycyclic 3-spirooxindoles bearing cyclopenta[b]indole units with four contiguous stereocenters in excellent yields and moderate to good stereoselectivities via a Michael/Friedel–Crafts/Mannich cascade.

 Please wait while we load your content...
Please wait while we load your content...