Copper-catalyzed electrophilic amination of sodium sulfinates at room temperature†
Abstract
By using O-benzoyl hydroxylamines as amine sources, the first convenient copper-catalyzed electrophilic amination of sodium sulfinates has been realized. Even with 2 mol% catalyst loading, the protocol provided an efficient and straightforward synthesis of a broad range of functional sulfonamides under ambient reaction conditions without an additional base and ligand. Based on the control experiments, a plausible mechanism was proposed.
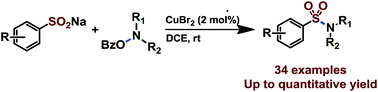

 Please wait while we load your content...
Please wait while we load your content...