NHC-catalyzed activation of α,β-unsaturated N-acyltriazoles: an easy access to dihydropyranones†
Abstract
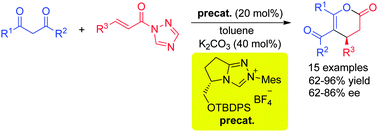
An N-heterocyclic carbene catalyzed activation of α,β-unsaturated N-acyltriazoles is described. The in situ generated α,β-unsaturated acylazolium intermediates allowed an enantioselective formal [3+3] cycloaddition with 1,3-dicarbonyl compounds. The resulting dihydropyranones are formed in good to excellent yields and with high enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...