Efficient heterocyclisation by (di)terpene synthases†
Abstract
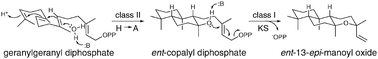
While cyclic ether forming terpene synthases are known, the basis for such heterocyclisation is unclear. Here it is reported that numerous (di)terpene synthases, particularly including the ancestral ent-kaurene synthase, efficiently produce isomers of manoyl oxide from the stereochemically appropriate substrate. Accordingly, such heterocyclisation is easily accomplished by terpene synthases. Indeed, the use of single residue changes to induce production of the appropriate substrate in the upstream active site leads to efficient bifunctional enzymes producing isomers of manoyl oxide, representing novel enzymatic activity.

 Please wait while we load your content...
Please wait while we load your content...