Direct gem-difluoromethylenation of sp3-hybridized carbon center through copper-mediated radical/radical cross-coupling for the construction of a CH2–CF2 linkage†
Abstract
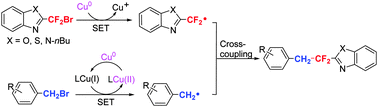
Efficient direct gem-difluoromethylenation of an sp3-hybridized carbon center in benzyl bromides using benzo-1,3-azolic (oxa-, thia- or aza-) difluoromethyl bromides for construction of a CH2–CF2 linkage has been developed through radical/radical C–C cross-coupling via two separate single electron transfer processes (SET) under the promotion of different copper sources.

 Please wait while we load your content...
Please wait while we load your content...