Homochiral metal phosphonate nanotubes†
Abstract
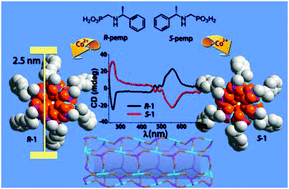
A new type of homochiral metal–organic nanotubular structures based on metal phosphonates are reported, namely, (R)- or (S)-[M(pemp)(H2O)2][M = CoII (1), NiII (2)] [pemp2− = (R)- or (S)-(1-phenylethylamino)methylphosphonate]. In these compounds, the tube-walls are purely inorganic, composed of metal ions and O–P–O bridges. The cavity of the nanotube is hydrophilic with one coordination water pointing towards the center, while the outer periphery of the nanotube is hydrophobic, decorated by the phenylethyl groups of pemp2−. The thermal stabilities, adsorption and proton conductivity properties are investigated.

 Please wait while we load your content...
Please wait while we load your content...