Highly selective copper-catalyzed trifunctionalization of alkynyl carboxylic acids: an efficient route to bis-deuterated β-borylated α,β-styrene†
Abstract
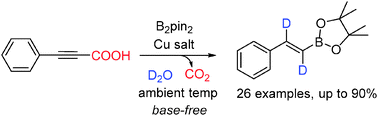
A copper-catalyzed highly efficient protocol for the synthesis of bis-deuterated β-borylated α,β-styrene derivatives, which can be further transformed to practical isotopically labeled compounds, has been developed. Alkynyl carboxylic acids are employed as alkyne synthons yet demonstrate a sharp discrepancy in reactivity and selectivity compared to terminal alkynes. Meanwhile, this reaction offers a novel and efficient strategy for highly selective trifunctionalization of the carbon–carbon triple bond at ambient temperature.

 Please wait while we load your content...
Please wait while we load your content...