Iron-catalyzed direct α-arylation of ethers with azoles†
Abstract
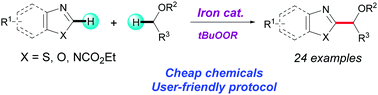
The direct α-arylation of cyclic and acyclic ethers with azoles has been achieved, which features a novel iron-catalyzed cross-dehydrogenative coupling (CDC) process. This practical oxidative method allowed the efficient C2-alkylation of a variety of (benzo)azoles constituting straightforward access to heterocycles of utmost medicinal significance and highlighting the convenient use of feedstock substrates and iron catalysts. A preliminary mechanism supported by DFT calculations is discussed as well.

 Please wait while we load your content...
Please wait while we load your content...