Diastereoselective Johnson–Corey–Chaykovsky trifluoroethylidenation†
Abstract
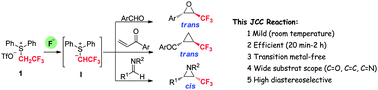
(2,2,2-Trifluoroethyl)diphenylsulfonium triflate was found to be an efficient ylide reagent for the Johnson–Corey–Chaykovsky reaction to afford trifluoromethyl epoxides, cyclopropanes and aziridines. Interestingly, excellent but different diastereoselectivity was observed for these transformations. Both trifluoromethyl epoxides and cyclopropanes were obtained with trans-selectivity, whereas aziridines were obtained with cis-selectivity.

 Please wait while we load your content...
Please wait while we load your content...