Catalyst-controlled divergence in cycloisomerisation reactions of N-propargyl-N-vinyl sulfonamides: gold-catalysed synthesis of 2-sulfonylmethyl pyrroles and dihydropyridines†
Abstract
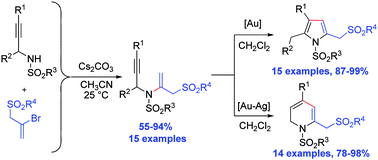
Gold-catalysed, divergent synthesis of 2-sulfonylmethyl pyrroles and dihydropyridines from N-propargyl-N-vinyl sulfonamides has been achieved. Echavarren's gold(I) catalyst promoted the formation of pyrrole derivatives whereas the combination of PPh3AuCl and AgSbF6 afforded dihydropyridines. The aza-enyne precursors for the cycloisomerisation reaction were prepared by a base-mediated formal vinylic substitution reaction of 2-bromoallyl sulfones.

 Please wait while we load your content...
Please wait while we load your content...