The amido-pentadienoate-functionality of the rakicidins is a thiol reactive electrophile – development of a general synthetic strategy†
Abstract
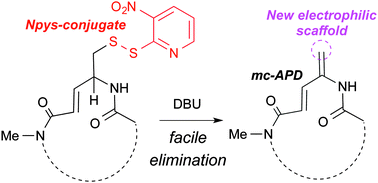
We demonstrate that a unique class-defining functionality (mc-APD) found in macrocyclic natural products with potent anti-cancer activity, imparts these compounds with electrophilic reactivity. The mc-APD group represents an interesting structural hybrid between canonical biologically relevant Michael-acceptors. Further, a novel thiol-elimination method for preparation of the mc-APD group is outlined.

 Please wait while we load your content...
Please wait while we load your content...