An iodine(iii) mediated oxidative rearrangement of enamines: efficient synthesis of α-amino ketones†
Abstract
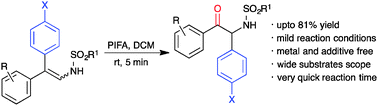
An iodine(III)-mediated, group-selective oxidative rearrangement of β,β-diarylenamines to α-amino ketones has been accomplished with excellent yield. The developed reaction involves the initial oxidation of enamine to an α-acyloxyimine intermediate and concomitant semipinacol rearrangement.

 Please wait while we load your content...
Please wait while we load your content...