The N,N′-dioxide/Ni(ii)-catalyzed asymmetric inverse-electron-demand hetero-Diels–Alder reaction of methyleneindolinones with hetero-substituted alkenes†
Abstract
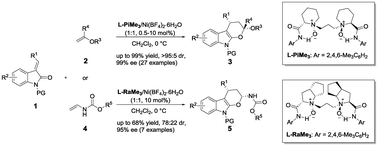
The highly efficient catalytic asymmetric inverse-electron-demand hetero-Diels–Alder reaction of methyleneindolinones with hetero-substituted alkenes has been accomplished under mild reaction conditions. In the presence of chiral N,N′-dioxide/Ni(II) complexes, a wide range of optically active dihydropyran-fused indoles were obtained in up to 99% yield, >95 : 5 dr and 99% ee.

 Please wait while we load your content...
Please wait while we load your content...