Complex transition metal hydrides: linear correlation of countercation electronegativity versus T–D bond lengths†
Abstract
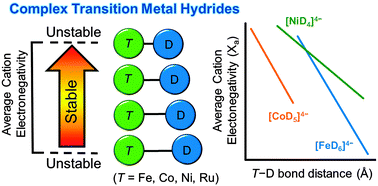
For homoleptic 18-electron complex hydrides, an inverse linear correlation has been established between the T–deuterium bond length (T = Fe, Co, Ni) and the average electronegativity of the metal countercations. This relationship can be further employed towards aiding structural solutions and predicting physical properties of novel complex transition metal hydrides.

 Please wait while we load your content...
Please wait while we load your content...