Construction of an all-carbon quaternary stereocenter by organocatalytic enantioselective α-functionalization of α-substituted β-ketocarbonyls with electron deficient vinylarenes†
Abstract
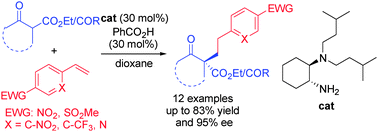
A chiral amine catalyzed enantioselective α-functionalization of α-substituted β-ketocarbonyls with electron-deficient vinylarenes has been developed to construct the dicarbonyl products with the formation of a chiral all-carbon quaternary stereocenter. The products can be used for the efficient synthesis of useful but challenging chiral quaternary centered pyrazolones.

 Please wait while we load your content...
Please wait while we load your content...