A detachable ester bond enables perfect Z-alkylations of olefins for the synthesis of tri- and tetrasubstituted alkenes†
Abstract
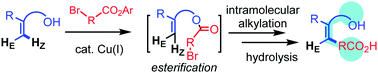
2-Vinyl-substituted phenol and an alpha-bromoester undergo a tandem esterification–alkylation reaction in the presence of a Cu–amine catalyst system to produce benzene-fused lactone. Z-Alkylated styrene is obtained after hydrolysis of the lactone with perfect selectivity. The simple protocol developed in this work opens a new avenue in the multi-substitution chemistry of alkenes.

 Please wait while we load your content...
Please wait while we load your content...