Photoinduced reductive perfluoroalkylation of phosphine oxides: synthesis of P-perfluoroalkylated phosphines using TMDPO and perfluoroalkyl iodides†
Abstract
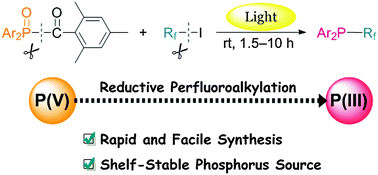
A photoinduced reaction between TMDPO (diphenyl(2,4,6-trimethylbenzoyl)-phosphine oxide) and perfluoroalkyl iodides successfully affords P-(perfluoroalkyl)diphenylphosphines as promising ligands for recyclable catalysts. Interestingly, the perfluoroalkylation reaction involves the reduction of phosphorus(V) compounds to phosphorus(III) species. The advantages of the present reaction include the use of an air-stable phosphorus source and good yields of P-perfluoroalkylphosphines in short reaction times.

 Please wait while we load your content...
Please wait while we load your content...