Synthesis of benzothiophene-based hydroxamic acids as potent and selective HDAC6 inhibitors†
Abstract
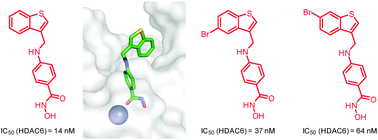
A small library of 3-[(4-hydroxycarbamoylphenyl)aminomethyl]benzothiophenes was prepared and assessed as a novel class of HDAC6 inhibitors, leading to the identification of three representatives as potent and selective HDAC6 inhibitors. Further tests with regard to inflammatory responses indicated that HDAC6 inhibition can be uncoupled from transcriptional inhibition at the level of activated NF-κB, AP-1, and GR.

 Please wait while we load your content...
Please wait while we load your content...