A stable bidentate protein binder achieved via DNA self-assembly driven ligand migration†
Abstract
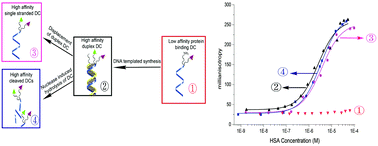
Herein we disclose the development of two complementary single stranded DNA-small molecule chimeras (DCs) that by themselves only bind weakly to a protein target (human serum albumin; HSA). However, upon self-assembly, the DC duplex facilitates a ligand migration reaction leading to a covalently fastened high-affinity, bidentate, protein-binder that resides at the terminus of only one of the DC strands. Due to this specific localization, the bidentate projection remains intact—and thus the system continues to strongly bind HSA—even under conditions that denature and degrade the DNA scaffolds.

 Please wait while we load your content...
Please wait while we load your content...