N-Heterocyclic carbene-catalyzed diastereoselective synthesis of β-lactone-fused cyclopentanes using homoenolate annulation reaction†
Abstract
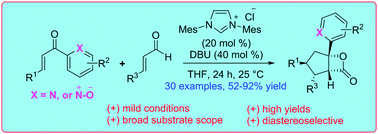
NHC-catalyzed annulation of enals with 2-enoylpyridines or 2-enoylpyridine N-oxides leading to the diastereoselective synthesis of β-lactone-fused cyclopentanes is reported. The reaction proceeds via the generation of homoenolate equivalent intermediates and tolerates a broad range of functional groups.

 Please wait while we load your content...
Please wait while we load your content...