Gold-catalyzed selective oxidation of 4-oxahepta-1,6-diynes to 2H-pyran-3(6H)-ones and chromen-3(4H)-ones via β-gold vinyl cation intermediates†
Abstract
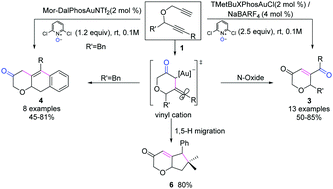
α-Oxo gold carbenes generated in situ via the gold-catalyzed selective oxidation of 4-oxahepta-1,6-diynes were effectively trapped by internal alkynes, resulting in the formation of 2H-pyran-3(6H)-ones, 3, and chromen-3(4H)-ones, 4, upon facile trapping the vinyl cation intermediates by an external N-oxide and internal aryl ring system.

 Please wait while we load your content...
Please wait while we load your content...