Titanium migration driven by Li vacancies in Li1−xTi2O4 spinel†
Abstract
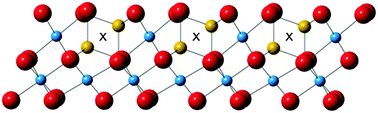
Gentle oxidation of lithium titanate spinel (LiTi2O4) with water at room temperature gives Li-deficient Li0.33Ti2O4. Combined X-ray and neutron Rietveld analysis shows that 28% of the Ti cations are displaced to alternative octahedral sites, in keeping with a proposed model based on Ti-migration limited by Li-vacancy concentration.

 Please wait while we load your content...
Please wait while we load your content...