Pd-catalyzed carbonylative access to aroyl phosphonates from (hetero)aryl bromides†
Abstract
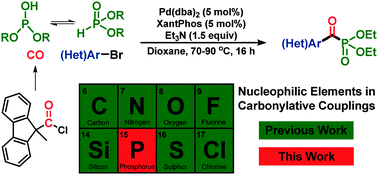
The first transition-metal catalysed carbonylation with a phosphorus nucleophile is presented. This transformation provides efficient and mild access to aroylphosphonates under mild conditions, thus ensuring a broad substrate scope. The utility of aroylphosphonates as useful reagents, capable of participating in a number of transformations, is subsequently demonstrated. Furthermore, access to [13C]-carbonyl labelled aroylphosphonates is easily realised for the first time, as only a near stoichiometric amount of CO is required applying this carbonylation.

 Please wait while we load your content...
Please wait while we load your content...