A new protocol for nickel-catalysed regio- and stereoselective hydrocyanation of allenes†
Abstract
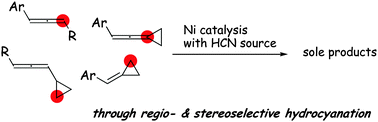
Regio- and stereoselective hydrocyanation under nickel catalysis is described. This report shows that allenyl C–C double bonds are discriminated and converted to the corresponding carbonitriles as a single product. The key functionalities for achieving high regio- and stereocontrol are aryl and cyclopropyl groups in the substrates.

 Please wait while we load your content...
Please wait while we load your content...