Peroxide promoted tunable decarboxylative alkylation of cinnamic acids to form alkenes or ketones under metal-free conditions†
Abstract
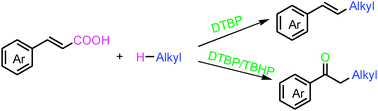
A tunable decarboxylative alkylation of cinnamic acids with alkanes was developed to form alkenes or ketones under transition metal-free conditions. In the presence of DTBP or DTBP/TBHP, the reaction gave alkenes and ketones respectively via a radical mechanism in moderate to good yields.

 Please wait while we load your content...
Please wait while we load your content...