Highly enantioselective copper(i)-catalyzed conjugate addition of 1,3-diynes to α,β-unsaturated trifluoromethyl ketones†
Abstract
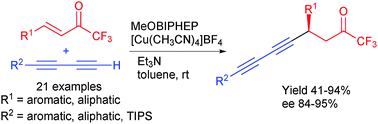
The conjugate diynylation of α,β-unsaturated trifluoromethyl ketones is carried out in the presence of a low catalytic load (2.5 mol%) of a copper(I)–MeOBIPHEP complex, triethylamine and a terminal 1,3-diyne. Pre-metalation of the terminal 1,3-diyne with stoichiometric or higher amounts of dialkylzinc reagent is not required. The corresponding internal diynes bearing a propargylic stereogenic center are obtained with good yields and excellent enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...