A formal anti-Markovnikov hydroamination of allylic alcohols via tandem oxidation/1,4-conjugate addition/1,2-reduction using a Ru catalyst†
Abstract
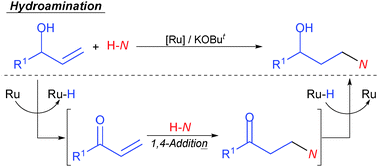
A formal anti-Markovnikov hydroamination of allylic alcohols using a Ru catalyst via tandem oxidation/1,4-conjugate addition/1,2-reduction was developed. Thus, the reaction of allylic alcohols with amines was performed in the presence of the catalyst generated from RuClH(CO)(PPh3)3 and 2,6-bis(n-butyliminomethyl)pyridine in situ to afford the corresponding γ-amino alcohols efficiently.

 Please wait while we load your content...
Please wait while we load your content...