The flavan–isoflavan rearrangement: bioinspired synthetic access to isoflavonoids via 1,2-shift–alkylation sequence†
Abstract
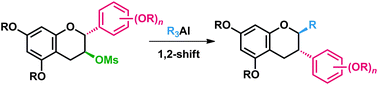
An approach to 2-substituted isoflavonoids is reported based on the 1,2-shift of the aryl group in the catechin skeleton followed by the in situ alkylation. Synthesis of (−)-equol, a natural isoflavan with estrogenic activities, was achieved.

 Please wait while we load your content...
Please wait while we load your content...