Acceleration of thiol additive-free native chemical ligation by intramolecular S → S acyl transfer†
Abstract
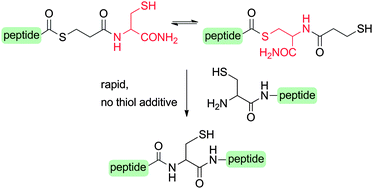
Peptide–mercaptopropionylcysteine (MPA–Cys) thioesters show a surprisingly high reactivity in native chemical ligation (NCL) and allow thiol-additive free reactions. This facilitates sequential NCL reactions and ligation–desulfurization reactions in one-pot formats. The synthetic utility is demonstrated by the synthesis of a SH3 domain.

 Please wait while we load your content...
Please wait while we load your content...