Near-infrared-induced electron transfer of an uranyl macrocyclic complex without energy transfer to dioxygen†
Abstract
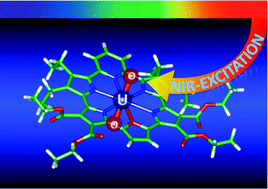
Photoexcitation of dichloromethane solutions of an uranyl macrocyclic complex with cyclo[1]furan[1]pyridine[4]-pyrrole (1) at the near-infrared (NIR) band (1177 nm) in the presence of electron donors and acceptors resulted in NIR-induced electron transfer without producing singlet oxygen via energy transfer.

 Please wait while we load your content...
Please wait while we load your content...