Using Li+ as the electrochemical messenger to fabricate an aqueous rechargeable Zn–Cu battery†
Abstract
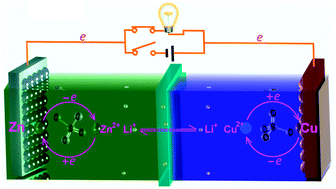
We propose an aqueous rechargeable Zn–Cu Daniell-type battery. In this system, Li+ prefers to conduct currents rather than react with the electrodes, while the Zn–Cu electrode couples engage in their electrochemical reactions free from conducting currents. Here Li+ performs like a messenger and thus could be called the electrochemical messenger.

 Please wait while we load your content...
Please wait while we load your content...