Regioselective annulation of nitrosopyridine with alkynes: straightforward synthesis of N-oxide-imidazopyridines†
Abstract
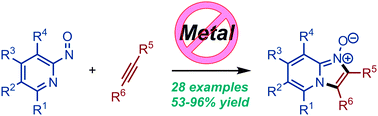
We have developed a novel method for the regioselective annulation of 2-nitrosopyridines with variably substituted alkynes under mild reaction conditions. This approach allows the annulation of alkynes with 2-nitrosopyridines under reagent- and catalyst-free reaction conditions. The developed method shows excellent functional group tolerance and provides easy access to N-oxide-imidazo[1,2-a]pyridines.

 Please wait while we load your content...
Please wait while we load your content...