Oxidative Heck desymmetrisation of 2,2-disubstituted cyclopentene-1,3-diones†
Abstract
Oxidative Heck couplings have been successfully developed for 2,2-disubstituted cyclopentene-1,3-diones. The direct coupling onto the 2,2-disubstituted cyclopentene-1,3-dione core provides a novel expedient way of enantioselectively desymmetrising all-carbon quaternary centres.
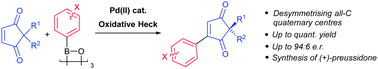

 Please wait while we load your content...
Please wait while we load your content...