Palladium-catalyzed picolinamide-directed coupling of C(sp2)–H and C(sp2)–H: a straightforward approach to quinolinone and pyridone scaffolds†
Abstract
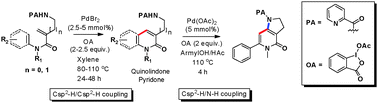
An unprecedented palladium-catalyzed picolinamide-directed coupling of C(sp2)–H and C(sp2)–H has been developed with exclusive formation of the six-membered ring heterocyclics – quinolinone and pyridone. The method employs cyclic hypervalent iodine as oxidant and features good functional-group tolerance. Another advantage of this reaction is that sequential C–H/C–H and C–H/N–H coupling could be achieved.

 Please wait while we load your content...
Please wait while we load your content...