Copper-catalyzed intermolecular and regioselective aminofluorination of styrenes: facile access to β-fluoro-N-protected phenethylamines†
Abstract
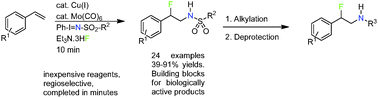
A copper-catalyzed regio- and intermolecular aminofluorination of styrenes has been developed. In this reaction Ph-I![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-Ts and Et3N·3HF act as nitrogen and fluorine sources, respectively. The obtained β-fluoro-N-Ts-phenethylamines can be N-alkylated with subsequent deprotection affording the corresponding β-fluoro-N-alkylated phenethylamines, which are interesting building blocks for compounds acting on neuronal targets.
N-Ts and Et3N·3HF act as nitrogen and fluorine sources, respectively. The obtained β-fluoro-N-Ts-phenethylamines can be N-alkylated with subsequent deprotection affording the corresponding β-fluoro-N-alkylated phenethylamines, which are interesting building blocks for compounds acting on neuronal targets.


 Please wait while we load your content...
Please wait while we load your content...