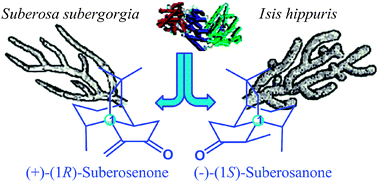
First enantioselective total synthesis and configurational assignments of suberosenone and suberosanone as potential antitumor agents†
Abstract
The first enantioselective total syntheses of two marine sesquiterpenes (1R)-suberosenone and (1R)-suberosanone are achieved leading to revision of the AC of natural (1S)-suberosanone. Key elements of the synthesis include hyperbaric asymmetric Michael addition and highly efficient silver trifluoroacetate mediated α-alkylation for the formation of ring A.


 Please wait while we load your content...
Please wait while we load your content...