Abstract
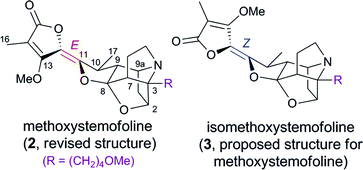
The first enantioselective total synthesis of (+)-methoxystemofoline (2) and (+)-isomethoxystemofoline (3) has been reported. The synthesis employed the halide-assisted bromotropanonation method that we developed recently to construct the core structure, and Overman's strategy for the implementation of the butenolide moiety. Through this work, the structure of methoxystemofoline was revised as 2 with an E-alkene, and its absolute configuration was established.

 Please wait while we load your content...
Please wait while we load your content...