A novel aqueous lithium–oxygen cell based on the oxygen-peroxide redox couple†
Abstract
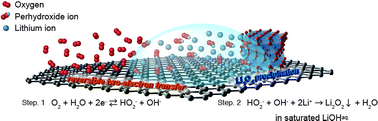
The electrochemical process of an aqueous Li–O2 cell is investigated. Li2O2 is detected as a discharge product of an aqueous Li–O2 cell using a catalyst-free carbon-based electrode. The electrolyte solution saturated with lithium hydroxide prevents hydrolysis of the Li2O2. Since the electron transfer process is based on the oxygen-peroxide redox couple, the galvanostatic charging–discharging profile shows stable cycling with an extremely low charging overpotential of <0.1 V at 1.0 mA cm−2.

 Please wait while we load your content...
Please wait while we load your content...