Diboron as a reductant for nickel-catalyzed reductive coupling: rational design and mechanistic studies†
Abstract
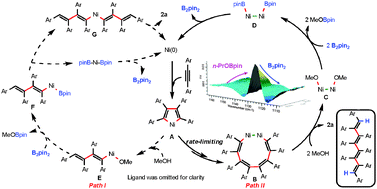
Diboron (B2pin2) has been identified as an efficient and environmentally benign reducing reagent for reductive coupling reactions for the first time, which enables the nickel-catalyzed reductive tetramerization of alkynes to be performed with high efficiency. Mechanistic and kinetic studies indicate that the facile reductive elimination to form the B–B bond from the dinuclear Ni–Ni complexes is responsible for the high efficiency. The activation enthalpy (ΔH‡ = 56.5 kJ mol−1), entropy (ΔS‡ = −128 J mol−1 K−1) and the substituent effect (ρ = 1.43) on this reaction were obtained.

 Please wait while we load your content...
Please wait while we load your content...