Electrochemical hydrogenation of a homogeneous nickel complex to form a surface adsorbed hydrogen-evolving species†
Abstract
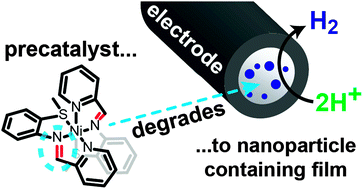
A Ni(II) complex degrades electrochemically in the presence of acid in acetonitrile to form an electrode adsorbed film that catalytically evolves hydrogen. Comparison with a similar compound permitted investigation of the degradation mechanism.
- This article is part of the themed collection: 2015 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...