Iron-mediated Markovnikov-selective hydro-trifluoromethylthiolation of unactivated alkenes†
Abstract
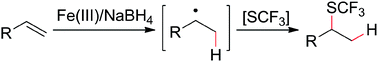
An iron-mediated hydro-trifluoromethylthiolation of unactivated olefins under mild conditions was described for the first time. The reaction occurred to full conversion within 30 min at 0 °C and tolerates a variety of functional groups. Preliminary mechanistic studies suggested that the reaction proceeds via a free-radical pathway.
- This article is part of the themed collection: 2015 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...